Conference Summaries
Lesser eGFR decline with dulaglutide regardless of weight changes in people with type 2 diabetes and moderate to severe chronic kidney disease (AWARD-7)
Presented by:Many antihyperglycaemic drugs, including insulin, are primarily cleared by the kidneys, restricting treatment options for patients with kidney disease. Dulaglutide is a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist that is not cleared by the kidneys, and confers a lower risk of hypoglycaemia than insulin. The AWARD-7 trial has shown that in patients with type 2 diabetes (T2D) and moderate-to-severe chronic kidney disease, once-weekly dulaglutide produced glycaemic control similar to that achieved with insulin glargine, with reduced decline in estimated glomerular filtration rate (eGFR) and significant weight loss.
- This post-hoc analysis of AWARD-7 assessed if the smaller decline in eGFR observed with dulaglutide is related to body weight loss.
Type of study, patients, and inclusion criteria
- AWARD-7 was a multicentre, open-label trial performed at 99 sites in nine countries.
- Participants were randomly assigned (1:1:1) to once-weekly injectable dulaglutide 1.5 mg, once-weekly dulaglutide 0.75 mg, or daily insulin glargine as basal therapy, all in combination with insulin lispro, for 52 weeks.
- Eligible patients were adults with T2D and moderate-to-severe chronic kidney disease (stages 3-4), with an HbA1c of 7.5-10.5%, and who were being treated with insulin or insulin plus an oral antihyperglycaemic drug and taking a maximum tolerated dose of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker.
Patient population
- Total number of enrolees: 577.
- Duration of follow-up: 52 weeks.
Primary outcome measure
- HbA1c at 26 weeks, with a 0.4% non-inferiority margin.
Secondary outcome measures
- eGFR and urine albumin-to-creatinine ratio (UACR).
Main findings of AWARD-7
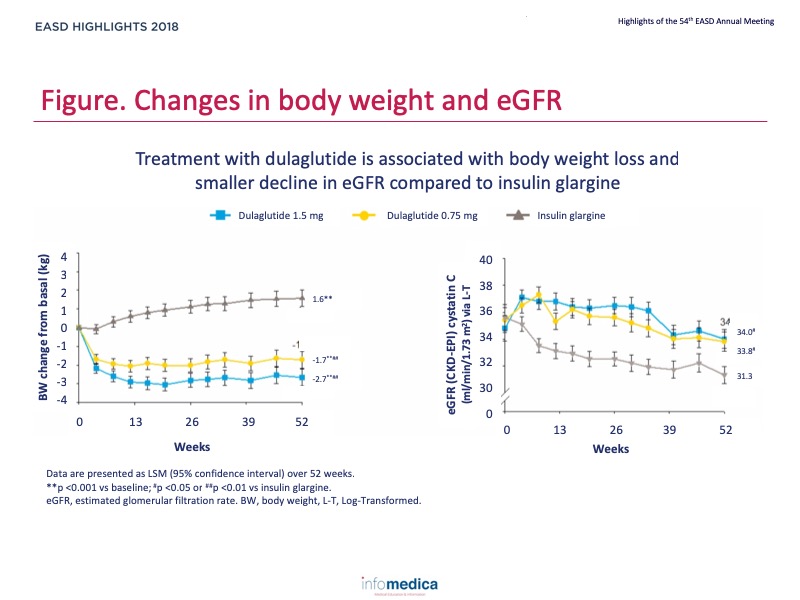
- Treatment with dulaglutide is associated with body weight loss and smaller decline in eGFR compared to insulin glargine (Figure).
Main findings of the post-hoc analysis
- There was no significant correlation between change in serum creatinine and change in body weight.
- There was no significant correlation between change in serum cystatin-C (a biomarker of kidney function which is not impacted by muscle mass) and change in body weight.
- There was no significant correlation between change in eGFR (CKD-EPI-cystatin C) and change in body weight.
- Treatment with dulaglutide is associated with body weight loss and smaller decline in eGFR compared to insulin glargine.
- In people with T2D and moderate to severe chronic kidney disease, dulaglutide was consistently associated with smaller eGFR decline compared to insulin glargine when serum creatinine or cystatin C were used to estimate eGFR.
- Changes in eGFR were independent of body weight changes; body weight loss or gain was not associated with greater changes in serum creatinine, cystatin C, or eGFR.
Key messages / Clinical Perspectives
- The beneficial finding of a smaller decline in eGFR observed with dulaglutide is independent of body weight loss.
REFERENCES
Present disclosure: Katherine R. Tuttle has reported relationships with Eli Lilly, Boehringer Ingelheim, AstraZeneca, and Gilead.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
