Conference Summaries
Cardiovascular and Renal Microvascular Outcome Study with Linagliptin in Patients with Type 2 Diabetes Mellitus (CARMELINA)
Presented by:Julio Rosenstock, MD
Dallas Diabetes Research Center, Dallas, TX, USA
Robert Toto, MD
University of Texas Southwestern Medical Center, Dallas, TX, USA
Steven E. Kahn, MD
Dept. of Medicine, University of Washington, Seattle, WA, USA
Darrn McGuire, MD
Dept.of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA
Vlado Perkovic, MD
Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
Mark Cooper, MD
Head of Diabetes, Monash University, Melbourne, VIC, Australia
Bernard Zinman, MD
University of Toronto, Toronto, Canada
Philip D. Homes, MD
Institute of Cellular Medicine, Diabetes, Newcastle University, Newcastle upon Tyne, UK
CARMELINA1 is a large, long-term cardiovascular (CV) outcomes trial testing the impact of linagliptin vs. placebo on top of standard care on CV and renal outcomes. The trial was designed to fulfil the linagliptin regulatory requirements for CV safety in a type 2 diabetes (T2D) population at high risk of CV risk enriched with chronic kidney disease (CKD). Based on its unique enriched CKD population, CARMELINA can add further evidence on the long-term safety of linagliptin on kidney function and heart failure.
- CARMELINA is investigating the long-term impact on cardiovascular morbidity, mortality and renal function of treatment with linagliptin in a selected population of patients with T2D and to compare outcomes against placebo, on a background of standard of care.
Type of study, patients, and inclusion criteria
- CARMELINA was an event driven, randomised, double-blind, placebo-controlled clinical trial conducted in 27 countries in T2D patients at high risk of CV and/or kidney events in which patients were randomised to linagliptin (5 mg/day) or placebo; medication was given on top of standard care.
- Key inclusion criteria included age ≥18 years, HbA1c 6.5-10%, BMI ≤45 kg/m2 and at high risk of CV disease due to either: 1) albuminuria (micro or macro) and macrovascular disease, and/or 2) impaired renal function with predefined UACR.
- CARMELINA was designed to continue until at least 611 participants had confirmed primary outcome events. Assuming a hazard ratio (HR) of 1.0, this provides 90% power to demonstrate non-inferiority of linagliptin versus placebo within the pre-specified non-inferiority margin of 1.3 at a one-sided α-level of 2.5%.
Patient population
- 6,979 participants randomised: 3,494 for placebo and 3,485 for linagliptin.
- Median time of study was 2.2 years with median treatment exposure of 1.9 years.
Primary outcome measure
- Time to the first occurrence of any of the following of the primary composite endpoint (3-point MACE): cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke.
Secondary outcome measures
- Time to the first occurrence of any of the following: composite renal endpoint (renal death, sustained end stage renal disease, sustained decrease of 40% or more in estimated glomerular filtration rate [eGFR]).
Baseline characteristics and metabolic endpoints
- At baseline, mean age was 66 years, HbA1c 8.0%, duration of T2D 15 years.
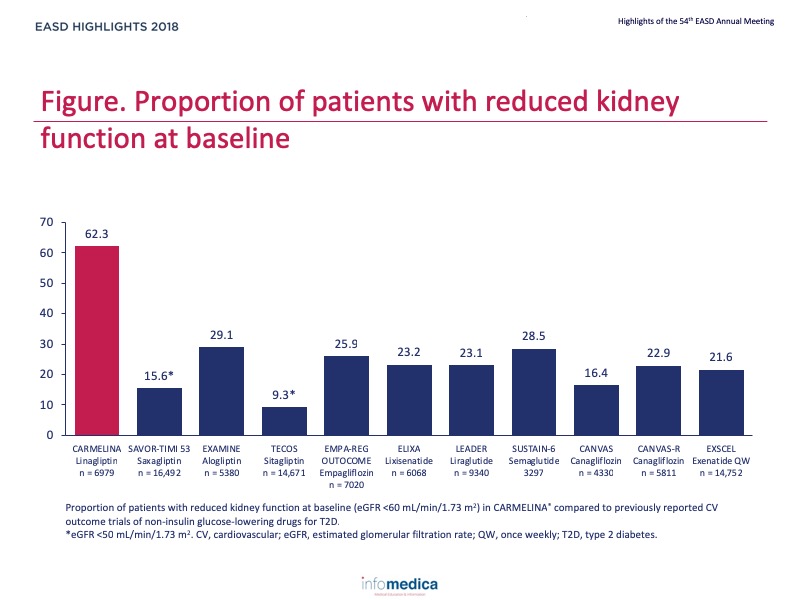
- 62% had eGFR <60 ml/min/1.73 m2.
- 80% had micro- or macroalbuminuria.
- By predefined criteria:
- 74% had prevalent CKD (eGFR <60 ml/min/1.73 m2 or macroalbuminuria)
- 57% had established CV disease
- 33% had both
- Mean difference in HbA1c across study was -0.36 favouring linagliptin.
- No between group differences in changes in body weight, lipids or blood pressure.
Cardiovascular outcomes
- Linagliptin did not increase the risk for 3 point-MACE (HR 1.02; 95% CI 0.89, 1.17).
- Linagliptin did not affect the risk for all-cause mortality (HR 0.98; 95% CI 0.84, 1.13).
- Linagliptin did not affect the risk of hospitalisation for heart failure (HR 0.90; 95% CI 0.84, 1.08).
Renal outcomes
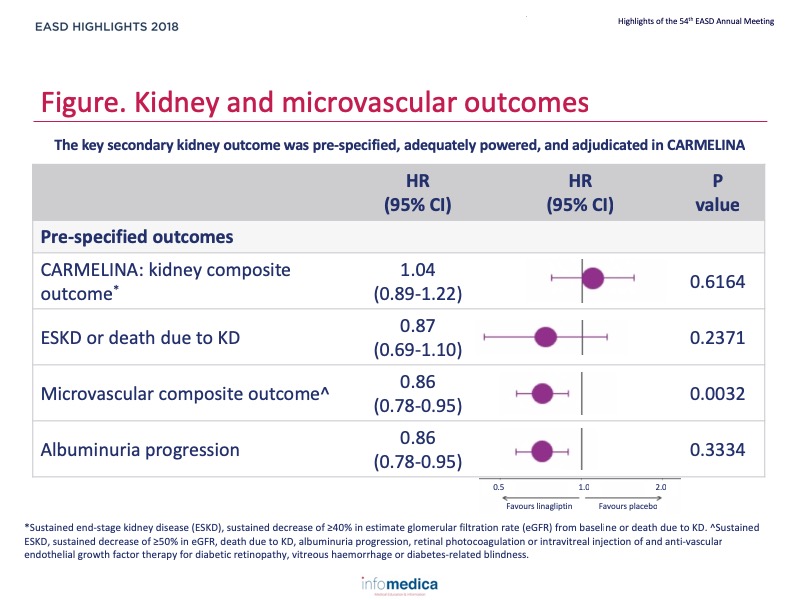
- Linagliptin did not affect the risk for sustained ≥40% reduction in eGFR, sustained end-stage kidney disease (ESKD), or death due to kidney disease (HR 1.04, 95% CI 0.89, 1.22).
- Linagliptin did not affect the risk for sustained ESKD or death due to kidney disease (HR 0.87; 95% CI 0.69, 1.10).
- Linagliptin significantly reduced the risk of progression to albuminuria (HR 0.86; 95% CI 0.78, 0.95; p = 0.0034).
- Linagliptin significantly reduced the risk for a microvascular composite endpoint (HR 0.86; 95% CI 0.78, 0.95; p = 0.0032).
- Figure, Table.
Hypoglycaemia and safety
- The safety findings were consistent with the known safety profile of linagliptin.
- Total adverse events, serious adverse events and adverse events leading to discontinuation occurred in similar numbers of patients in each group.
- Investigator-reported hypoglycaemia (including severe hypoglycaemia) occurred in similar numbers of patients in each group.
- Consistent with the label of linagliptin, acute pancreatitis and pemphigoid events were rare but more frequent with linagliptin than placebo.
- In CARMELINA, linagliptin demonstrated CV safety in a clinically-relevant population of subjects with T2D, CV disease and CKD.
- Linagliptin did not increase hospitalisation for heart failure, even in those at high risk of heart failure.
- The overall safety profile, including kidney outcomes, in this high-risk population is reassuring.
Key messages/Clinical Perspectives
- In the high-risk population of CARMELINA, linagliptin demonstrated CV safety for atherosclerotic CV events, and with neutral effects on hospitalisation for heart failure and kidney outcomes.
REFERENCES
Presenter disclosure: Julio Rosenstock has served on scientific advisory boards and receive honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia; he has also received grants/researcher support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genetech, Janssen, Lexicon, Boehringer Ingelheim, and Intarcia.Robert Toto reports consultant fees from Amgen, Boehringer Ingelheim, ZS Pharma, Relypsa, Novo Nordisk, Reata, and AstraZeneca, and receives grant support from the NIH.Steven Kahn reports fees for advisory (advisory boards, steering committees) service to Boehringer Ingelheim, Elceylx, Intarcia, Janssen, Merck, Novo Nordisk.Darren McGuire reports clinical trial leadership for Merck AstraZeneca, Janssen, Eli Lilly, Boehringer Ingelheim, Novo Nordisk, Lexicon, Eisai, GlaxoSmithKline, Sanof Aventis; consultancy for Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Merck, AstraZeneca, Metavant, Applied Therapeutics.Vlado Perkovic reports consults for Abbvie, Astellas, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Retrophin, and AstraZeneca; has received lecture fees or grant support from Baxter, Boehringer Ingelheim, Merck, and Pfizer; and his institution has held clinical trial contracts with Abbvie, Roche, Janssen, Servier, and Novartis.Mark Cooper reports fee for advisory services from Boehringer Ingelheim.Bernard Zinman reports grant support from Boehringer Ingelheim, AstraZeneca, and Novo Noridsk; and consulting fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Noridsk, and Sanofi Aventis.Philip D. Homes has reported that no relationships exist relevant to the contents of this presentation.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
