Conference Summaries
Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial
Presented by:Erin A. Bohula, MD
Benjamin M. Scirica, MD
Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA
There is a direct relationship between bodyweight and risk of diabetes. Lorcaserin, a selective serotonin 2C receptor agonist that suppresses appetite, has been shown to facilitate sustained weight loss in obese or overweight patients.
- CAMELLIA-TIMI 61 evaluated the long-term effects of lorcaserin on diabetes prevention and remission.
Type of study, patients and inclusion criteria
- Randomised, double-blind, placebo-controlled trial.
- Patients were randomly assigned to receive either lorcaserin (10 mg twice daily) or matching placebo; all patients had access to a standardised weight management programme based on lifestyle modification.
Patient populations
- Total number of enrolees: 12,000.
- Duration of follow-up: median of 3.3 years.
- Eligible patients were aged 40 years or older; patients at high risk for atherosclerotic vascular disease had to be aged 50 years or older with diabetes and at least one other risk factor.
Primary outcome measures
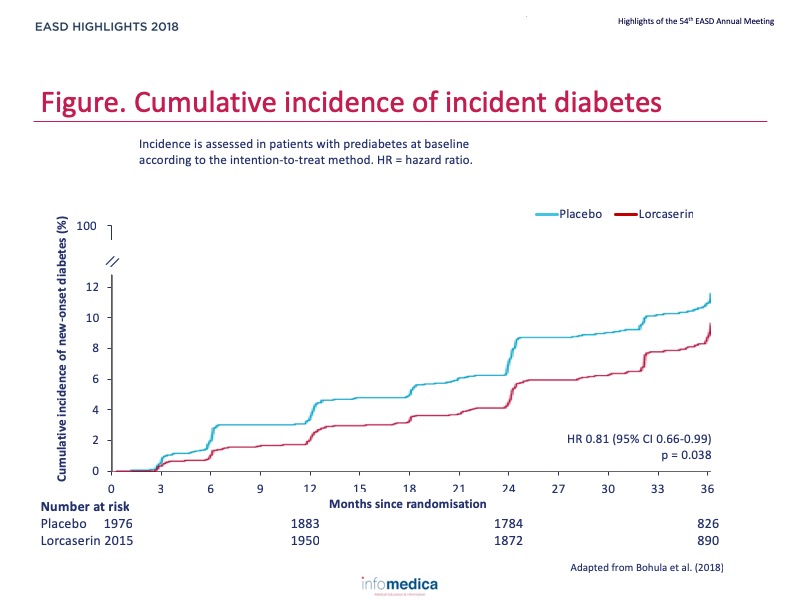
- The prespecified primary metabolic efficacy endpoint was time to incident diabetes, and assessed in patients with prediabetes at baseline.
Secondary outcome measures
- The prespecified secondary outcomes for efficacy were incident diabetes in all patients without diabetes, achievement of normoglycaemia in patients with prediabetes, and change in HbA1c in patients with diabetes.
- Hypoglycaemia was a prespecified safety outcome.
Primary endpoints or outcomes
- At baseline, 6816 patients (56.8%) had diabetes, 3991 (33.3%) prediabetes, and 1193 (9.9%) normoglycaemia.
- At 1 year, patients treated with lorcaserin had a net weight loss beyond placebo of 2.6 kg (95% CI 2.3-2.9) for those with diabetes, 2.8 kg (2.5-3.2) for those with prediabetes, and 3.3 kg (2.6-4.0) for those with normoglycaemia (p <0.0001 for all analyses).
- Lorcaserin reduced the risk of incident diabetes by 19% in patients with prediabetes (172 [8.5%] of 2015 vs 204 [10.3%] of 1976; hazard ratio 0.81, 95% CI 0.66-0.99; p = 0.038) and by 23% in patients without diabetes (174 [6.7%] of 2615 vs 215 [8.4%] of 2569; 0.77, 0.63-0.94; p = 0.012) (Figure).
Secondary endpoints or outcomes
- Lorcaserin resulted in a non-significant increase in the rate of achievement of normoglycaemia in patients with prediabetes (185 [9.2%] vs 151 [7.6%]; 1.20, 0.97-1.49; p = 0.093).
- In patients with diabetes, lorcaserin resulted in a reduction of 0.33% (95% CI 0.29-0.38; p<0.0001) in HbA1c compared with placebo at 1 year from a mean baseline of 53 mmol/mol (7.0%).
- In patients with diabetes at baseline, severe hypoglycaemia with serious complications was rare, but more common with lorcaserin (12 [0.4%] vs four [0.1%] events; p = 0.054).
- When added to lifestyle interventions, lorcaserin significantly reduced the incidence of diabetes, non-significantly increased the proportion of patients with prediabetes achieving normoglycaemia, significantly increased the proportion of patients with diabetes achieving remission of hyperglycaemia, and significantly reduced the risk of diabetic microvascular complications.
Key messages / Clinical Perspectives
- These findings reinforce the notion that modest, durable weight loss can improve cardiometabolic health and supports the role of lorcaserin as an adjunctive therapy in chronic weight management and metabolic health.
REFERENCES
Present disclosure: Erin A. Bohula reports grants from Eisai, during the conduct of the study; and personal fees from Servier, Merck, National Institutes OF Health, Lexicon, Medscape, Academic CME, MD Conference Express, Paradigm, and Novartis, and grants from Amgen, AstraZeneca, and Merck, outside of the submitted work. Benjamin M. Scirica reports grants from Eisai, during the conduct of the study; and grants from AstraZenaca, Novartis, and Merck; personal fees from AstraZeneca, Biogen Idec, Boehringer Ingelheim, Covance, Dr Reddy’s Laboratory, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Merck, NovoNordisk, Sanofi, and St Jude’s Medical; and other support from Health [at] Scale, outside of the submitted work.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
