Conference Summaries
Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (HARMONY Outcomes): a double-blind, randomised placebo-controlled trial
Presented by:Adrian F. Hernandez, MD
Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA
Jennifer B. Green, MD
Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA
Stefano Del Prato, MD
Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
John J.V. McMurray, MD
British Heart Foundation Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow G12 8TA, UK
Christopher B. Granger, MD
Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, USA
Lawrence A. Leiter, MD
Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada
Glucagon-like peptide 1 receptor agonists differ in chemical structure, duration of action and their effects on clinical outcomes. The cardiovascular (CV) effects of once-weekly albiglutide in type 2 diabetes are not well characterised.
- The HARMONY study investigated the safety and efficacy of albiglutide in preventing CV death, myocardial infarction (MI), or stroke.
Type of study, patients, and inclusion criteria
- Double-blind, randomised, placebo-controlled trial at 610 sites across 28 countries.
- Patients with type 2 diabetes and CV disease were randomised (at a 1:1 ratio) to receive either subcutaneous injection of albiglutide (30-50 mg, based on glycaemic response and tolerability) or a matched volume of placebo once a week, in addition to standard care.
- Key inclusion criteria ensured a high rate of CV events: established disease of the coronary (MI, at least 50% stenosis in one coronary artery or more, or previous coronary revascularisation), cerebrovascular disease (ischaemic stroke, at least 50% carotid artery stenosis, or a previous carotid vascular procedure), or peripheral artery disease (intermittent claudication and an ankle to brachial index <0.9, non-traumatic amputation, or a previous peripheral vascular procedure) who had a HbA1c level of more than 7.0% (53 mmol/mol).
Patient populations
- Total number of enrolees: 9463.
- Age: ≥40 years.
- Duration of follow-up: median of at least 1.6 years.
Primary outcome measure
- Composite of death from CV causes, MI, or stroke.
Secondary outcome measures
- Secondary CV outcomes: a four-component composite (the primary composite, with the addition of urgent revascularisation for unstable angina), the individual components of the primary endpoint, and the composite of CV death or hospital admission because of heart failure.
- Secondary metabolic outcomes: the time to initiation of chronic insulin therapy, the time to the first occurrence of an important microvascular event, changes in HbA1c and bodyweight, and the proportion of participants who attained glycaemic control without severe hypoglycaemia and who gained less than 5% of their bodyweight by the end of the study.
- Safety outcomes: the change in blood pressure and heart rate, change in eGFR, and adverse events of special interest, which included the development of prespecified malignancies (medullary thyroid cancer, pancreatic cancer, and haematological malignancies), pancreatitis, severe hypoglycaemia, injection site reactions, immunological reactions, diabetic retinopathy, worsening renal function, and death from any cause.
Primary endpoints or outcomes
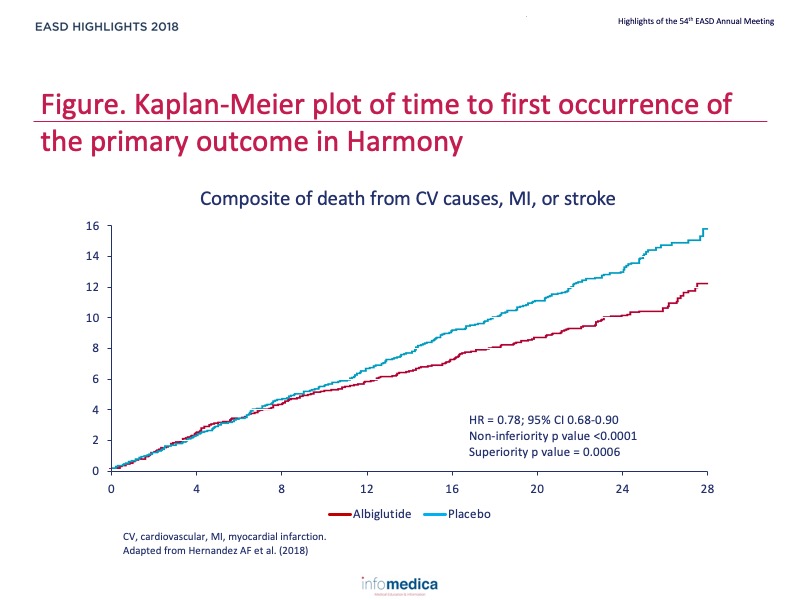
- The primary composite outcome occurred in 338 (7%) of 4731 patients at an incidence rate of 4.6 events per 100 person-years in the albiglutide group and in 428 (9%) of 4732 patients at an incidence rate of 5.9 events per 100 person-years in the placebo group (hazard ratio 0.78, 95% CI 0.68-0.90), which indicated that albiglutide was superior to placebo (p<0.0001 for noninferiority; p = 0.0006 for superiority) (Figure).
Secondary endpoints or outcomes
- The hazard ratios for each of the components of the composite primary endpoint were 0.93 (95% CI 0.73-1.19) for death from CV causes, 0.75 (0.61-0.90) for MI, and 0.86 (0.66-1.14) for stroke.
- The incidence of acute pancreatitis (10 patients in the albiglutide group and 7 patients in the placebo group), pancreatic cancer (6 patients in the albiglutide group and 5 patients in the placebo group), medullary thyroid carcinoma (no patients in either group), and other serious adverse events did not differ between the two groups.
- There were three (<1%) deaths in the placebo group that were assessed by investigators, who were masked to study drug assignment, to be treatment-related and two (<1%) deaths in the albiglutide group.
- In patients with type 2 diabetes and CV disease, albiglutide was superior to placebo with respect to major adverse CV events.
Key messages / Clinical Perspectives
- Certain GLP-1 receptor agonists reduce the risk of atherothrombotic events in patients with type 2 diabetes and high CV risk.
- Evidence-based GLP-1 receptor agonists should be considered as part of a comprehensive strategy to reduce the risk of CV events in patients with type 2 diabetes.
REFERENCES
Present disclosure: Adrian F. Hernandez reports grants to his institution from AstraZeneca, GlaxoSmithKline, Luitpold Pharmaceuticals, Novartis, Merck, Portola Pharmaceuticals, and Verily; and he has been a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Novartis, and Merck. Jennifer B. Green reports grants to her institution from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; and she has been consultant for AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Merck. SJ, NPJ, and KMT are GlaxoSmithKline employees and shareholders. Stefano Del Prato reports grants to his institution from AstraZeneca, Boehringer Ingelheim, Merck, and Novartis; he reports honoraria for presentations from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Merck, Novartis, Novo Nordisk, and Takeda Pharmaceuticals; and he reports participation in advisory boards for Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Merck, Mundipharma, Novartis Pharmaceuticals, Novo Nordisk, Sanofi, Servier, and Takeda Pharmaceuticals. John J.V. McMurray reports grants to his institution from Boehringer Ingelheim and Bristol-Myers Squibb, consultancy fees to his institution from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Cardurion Pharmaceuticals, DalCor Pharmaceuticals, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, and Theracos, and honoraria to his institution for presentations from AstraZeneca, Novartis, and Pfizer.
Christopher B. Granger reports grants to his institution from Apple, Armetheon, Daiichi Sankyo, the US Food and Drug Administration, and AstraZeneca; and reports consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen Pharmaceutica, Medtronic, the National Institutes of Health, Novartis, Pfizer, AbbVie, Boston Scientific, Gilead Sciences, Medscape, Merck, Novo Nordisk, Rho, Roche Diagnostics, Sirtex Medical, and Verseon.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
Trial sponsor: GlaxoSmithKline Research & Development (GSK).
Trial: NCT02465515
