Conference Summaries
Efficacy and safety of LY3298176, a novel dual GIP/GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial
Presented by:Juan Pablo Frias, MD
National Research Institute, Los Angeles, CA, USA
Combined treatment with GLP-1 and GIP receptor agonists could result in additive effects on glucose and bodyweight regulation. LY3298176 is a 39-amino acid synthetic peptide with agonist activity at both the GIP and GLP-1 receptors. Preclinical data showed that LY3298176 has a greater affinity to GIP relative to GLP-1 receptors expressed on cells.
- This phase 2 study aimed to explore the dose-response relationship of LY3298176 (1, 5, 10, and 15 mg once-weekly) in patients with type 2 diabetes (T2D) and collect initial efficacy and safety data in comparison with placebo and dulaglutide 1.5 mg once-weekly.
Type of study, patients, and inclusion criteria
- Phase 2b, randomised, double-blind study at 47 sites.
- Patients with T2D were randomly assigned (1:1:1:1:1:1) to receive either once-weekly subcutaneous LY3298176 (1 mg, 5 mg, 10 mg, or 15 mg), once-weekly dulaglutide (1.5 mg), or placebo for 26 weeks.
Patient populations
- Total number of enrolees: 318.
- Age: 18-75 years.
- Duration of follow-up: 26 weeks.
- Eligible participants (aged 18-75) had T2D for at least 6 months (HbA1c 7.0-10.5%, inclusive) that was inadequately controlled with diet and exercise alone or with stable metformin therapy for at least 3 months before screening, and BMI of 23-50 kg/m2.
Primary endpoint
- Change in HbA1c from baseline to 26 weeks in the modified intention-to-treat (mITT) population (all patients who received at least one dose of study drug and had at least one postbaseline measurement of any outcome).
Secondary outcome measures:
- Change in HbA1c from baseline to 12 weeks.
- Change in mean bodyweight, fasting plasma glucose, waist circumference, total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides.
- Proportion of patients reaching HbA1c target (≤6.5% and <7.0%) from baseline to weeks 12 and 26.
- Proportion of patients with at least 5% and 10% bodyweight loss from baseline to 26 weeks.
- Safety.
Primary endpoints or outcomes:
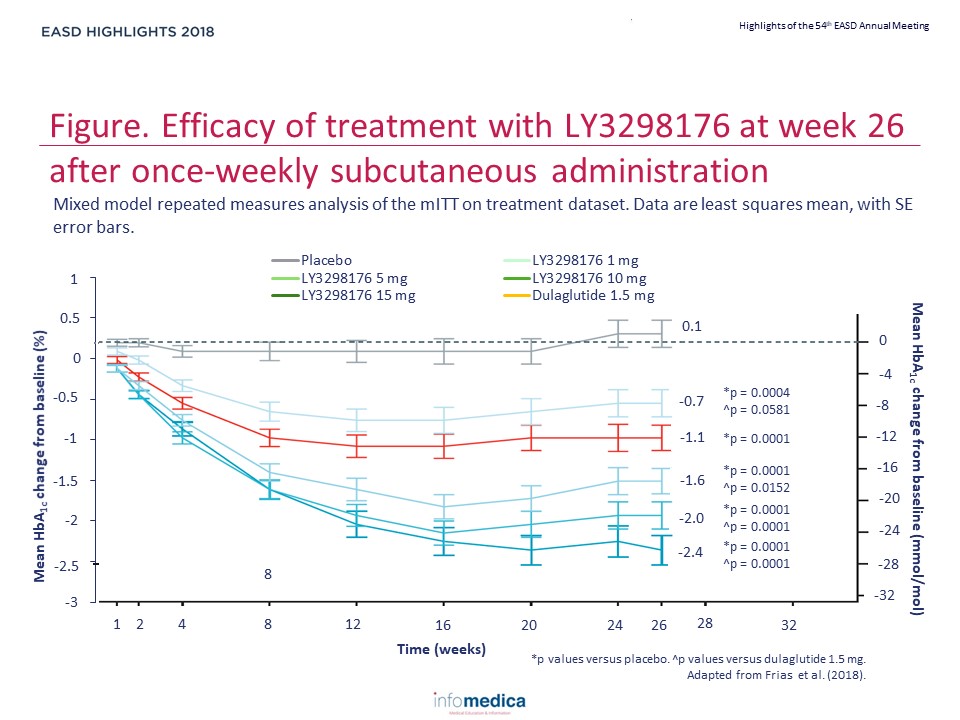
- At 26 weeks, the effect of LY3298176 on change in HbA1c was dose-dependent and did not plateau.
- Mean changes from baseline in HbA1c with LY3298176 were -1.06% for 1 mg, -1.73% for 5 mg, -1.89% for 10 mg, and -1.94% for 15 mg, compared with -0.06% for placebo (posterior mean differences [80% credible set] vs placebo: -1.00% [-1.22 to -0.79] for 1 mg, -1.67% [-1.88 to -1.46] for 5 mg, -1.83% [-2.04 to -1.61] for 10 mg, and -1.89% [-2.11 to -1.67] for 15 mg).
- Compared with dulaglutide (-1.21%) the posterior mean differences (80% credible set) for change in HbA1c from baseline to 26 weeks with the LY3298176 doses were 0.15% (-0.08 to 0.38) for 1 mg, -0.52% (-0.72 to -0.31) for 5 mg, -0.67% (-0.89 to -0.46) for 10 mg, and -0.73% (-0.95 to -0.52) for 15 mg.
- The results of the mixed-effect model for repeated measures in the mITT on treatment datasets were consistent with those of the analyses with the Bayesian model, and demonstrated greater reductions in HbA1c with 5 mg, 10 mg, and 15 mg LY3298176 compared with dulaglutide (p=0.0152 for 5 mg, p=0.0001 for 10 mg, p<0.0001 for 15 mg) (Figure).
Secondary endpoints or outcomes
- At 26 weeks, 33-90% of patients treated with LY3298176 achieved the HbA1c target of less than 7.0% (vs 52% with dulaglutide, 12% with placebo) and 15-82% achieved the HbA1c target of at least 6.5% (vs 39% with dulaglutide, 2% with placebo).
- Changes in fasting plasma glucose ranged from -0.4 mmol/L to -3.4 mmol/L for LY3298176 (vs 0.9 mmol/L for placebo, -1.2 mmol/L for dulaglutide).
- Changes in mean bodyweight ranged from -0.9 kg to -11.3 kg for LY3298176 (vs -0.4 kg for placebo, -2.7 kg for dulaglutide).
- At 26 weeks, 14-71% of those treated with LY3298176 achieved the weight loss target of at least 5% (vs 22% with dulaglutide, 0% with placebo) and 6-39% achieved the weight loss target of at least 10% (vs 9% with dulaglutide, 0% with placebo).
- Gastrointestinal events (nausea, diarrhoea and vomiting) were the most common treatment-emergent adverse events.
- The incidence of gastrointestinal events was dose-related (23.1% for 1 mg LY3298176, 32.7% for 5 mg LY3298176, 51.0% for 10 mg LY3298176, and 66.0% for 15 mg LY3298176, 42.6% for dulaglutide, 9.8% for placebo); most events were mild to moderate in intensity and transient.
- Decreased appetite was the second most common adverse event (3.8% for 1 mg LY3298176, 20.0% for 5 mg LY3298176, 25.5% for 10 mg LY3298176, 18.9% for 15 mg LY3298176, 5.6% for dulaglutide, 2.0% for placebo).
- There were no reports of severe hypoglycaemia.
- LY3298176, a novel dual GIP and GLP-1 receptor dual agonist, resulted in statistically significant and clinically meaningful control of HbA1c with greater weight loss and an acceptable tolerability profile, as compared with dulaglutide, a GLP-1 receptor agonist.
- LY3298176 has an acceptable safety and tolerability profile.
Key messages
- LY3298176 has the potential to become a treatment option for patients with T2D, and warrants further study of its efficacy and safety in a phase 3 programme with an optimised administration regimen.
REFERENCES
Present disclosure: The presenter has reports relationships for employment/consultancy with AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Gilead; and grants from El. Lilly.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
Trial sponsor: Eli Lilly
Trial: NCT03131687
