Conference Summaries
Update and new findings from the Restoring Insulin SEcretin (RISE) study
Presented by:Anny H. Xiang, MD
Kaiser Permanente Southern California, Pasadena, CA, USA
Thomas A. Buchanan, MD
Diabetes and Obesity Research Institute, University of Southern California, Los Angeles, CA, USA
Dept. of Medicine, Keck School of Medicine of USC, Los Angeles, CA, USA
Des preuves solides suggèrent que l’apparition du diabète serait liée à un perte de fonction des cellules ß. Les interventions visant à réduire la masse adipeuse ou atténuer ses effets sur le métabolisme sont celles qui permettent de retarder et/ou de prévenir le diabète de type 2 avec le plus de succès (DT2).
- The RISE study is determining whether medication or surgical intervention strategies can preserve β-cell function in adults and youth with prediabetes or early T2D. In particular, the effects of sustained weight loss following gastric banding in adults are presented.
Type of study, patients, and inclusion criteria
- ß-cell function was measured using hyperglycaemic clamps and oral glucose tolerance tests (OGTTs).
- Main inclusion criteria included age 21-65 years, BMI 30-40 kg/m2, fasting glucose >90 mg/dl, 2-hour OGTT glucose ≥140 mg/dl, HbA1c ≤7.0%, diabetes duration <1 year and treatment naive for diabetes medication.
- Participants were randomised 1:1 to gastric banding or metformin.
Patient population
- Total number of enrolees: 70.
- Duration of follow-up: 24 months.
Primary outcome measure
- Two co-primary outcomes were used to measure ß-cell responses:
- Steady-state C-peptide (SSCP)
- Acute C-peptide response at maximal glycaemia (ACPRmax).
Secondary outcome measures
- Glucose measure:
- OGTT fasting and 2-hour glucose
- OGTT glucose tolerance status
- HbA1c.
- Additional ß-cell measures.
- Blood pressure, lipids, liver enzymes.
- Adverse events.
Primary endpoints or outcomes
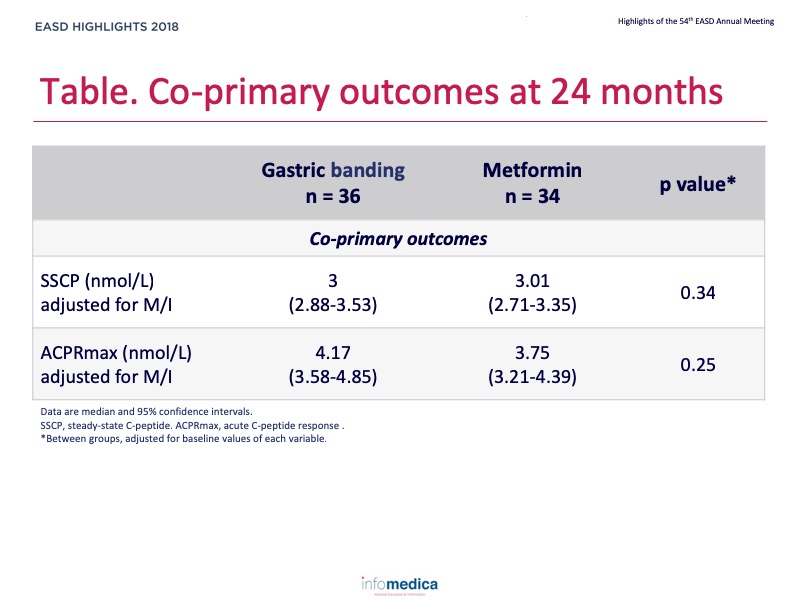
- There were no differences between the gastric banding and metformin groups at 24 months considering the primary outcomes (Table).
- There were no significant interactions between treatment groups and diabetes or impaired glucose tolerance (IGT) status for the co-primary outcomes.
Secondary endpoints or outcomes
- Body weight decreased significantly in the gastric banding group, but not in the metformin group, compared to baseline (mean 10.6 vs. 1.6 kg; p <0.05 between groups).
- There were no differences in clinical glucose status (normal, impaired, diabetes) between groups.
- ß-cell responses fell in a pattern that maintained relatively stable compensation for insulin resistance.
- Acute ß-cell compensation to glucose improved significantly with gastric banding.
- ß-cell compensation at maximal stimulation fell significantly with metformin.
- Glucose levels improved only slightly.
- Over 2 years in adults patients with mild-moderate obesity and IGT or recently diagnosed mild T2D:
- Gastric banding, but not metformin, was associated with significant and sustained weight loss
- Both gastric banding and metformin led to about 50% improvement in insulin sensitivity at one year, with attenuation of the effect at 2 years.
- Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild-moderate obesity and IGT or recently diagnosed mild T2D.
- The predominant ß-cell response was a reduction in secretion to maintain relatively constant compensation for insulin resistance, with only small improvements in glucose.
- Whether these interventions will have different effects on ß-cell function in the long term remains to be determined.
Type of study, patients, and inclusion criteria
- ß-cell function was measured using hyperglycaemic clamps and oral glucose tolerance tests (OGTTs).
- Main inclusion criteria included age 21-65 years, BMI 30-40 kg/m2, fasting glucose >90 mg/dl, 2-hour OGTT glucose ≥140 mg/dl, HbA1c ≤7.0%, diabetes duration <1 year and treatment naive for diabetes medication.
- Participants were randomised 1:1 to gastric banding or metformin.
Patient population
- Total number of enrolees: 70.
- Duration of follow-up: 24 months.
Primary outcome measure
- Two co-primary outcomes were used to measure ß-cell responses:
- Steady-state C-peptide (SSCP)
- Acute C-peptide response at maximal glycaemia (ACPRmax).
Secondary outcome measures
- Glucose measure:
- OGTT fasting and 2-hour glucose
- OGTT glucose tolerance status
- HbA1c.
- Additional ß-cell measures.
- Blood pressure, lipids, liver enzymes.
- Adverse events.
Primary endpoints or outcomes
- There were no differences between the gastric banding and metformin groups at 24 months considering the primary outcomes (Table).
- There were no significant interactions between treatment groups and diabetes or impaired glucose tolerance (IGT) status for the co-primary outcomes.
Secondary endpoints or outcomes
- Body weight decreased significantly in the gastric banding group, but not in the metformin group, compared to baseline (mean 10.6 vs. 1.6 kg; p <0.05 between groups).
- There were no differences in clinical glucose status (normal, impaired, diabetes) between groups.
- ß-cell responses fell in a pattern that maintained relatively stable compensation for insulin resistance.
- Acute ß-cell compensation to glucose improved significantly with gastric banding.
- ß-cell compensation at maximal stimulation fell significantly with metformin.
- Glucose levels improved only slightly.
- Over 2 years in adults patients with mild-moderate obesity and IGT or recently diagnosed mild T2D:
- Gastric banding, but not metformin, was associated with significant and sustained weight loss
- Both gastric banding and metformin led to about 50% improvement in insulin sensitivity at one year, with attenuation of the effect at 2 years.
Key messages / Clinical Perspectives
- Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild-moderate obesity and IGT or recently diagnosed mild T2D.
- The predominant ß-cell response was a reduction in secretion to maintain relatively constant compensation for insulin resistance, with only small improvements in glucose.
- Whether these interventions will have different effects on ß-cell function in the long term remains to be determined.
REFERENCES
Present disclosure: Anny H. Xiang has reported that no relationships exist relevant to the contents of this presentation. Thomas A. Buchanan has reported relationships for research support whit Allergan Corporation and Apollo Endosurgery.
Written by: Patrick Moore, PhD
Reviewed by: Marco Gallo, MD
